Abstract
Introduction
Peripheral neuropathies (PN) caused by immunoglobulin M (IgM) paraproteinemia are rare disorders that require close collaboration between hematologists and neurologists for adequate diagnosis and management. The IgM paraproteinemia is caused by an underlying B-cell clone that can often be classified as IgM monoclonal gammopathy (IgM monoclonal gammopathy of undetermined significance (MGUS) or Waldenström macroglobulinaemia (WM)). Anti-myelin-associated glycoprotein (MAG) IgM paraprotein related PN (anti-MAG PNP) is the most frequent variety and accounts for approximately 50% of cases. Treatment targeted at the B-cell clone i.e. rituximab +/- chemotherapy, is only indicated in severe and/or progressive cases. However, establishing disease severity and tracking progression over time is difficult since the most prominent symptoms, i.e. imbalance, gait ataxia, sensory disturbances and tremor, are difficult to capture reliably. Anti-MAG titers and paraprotein levels do not correlate well with disease activity and are thus not suitable as biomarkers for disease severity, activity and/or response. The lack of reliable biomarkers also impacts successful execution of clinical trials and therefore hampers introduction of novel targeted non-cytotoxic therapies such as BTK inhibition in this patient population. Therefore, biomarkers for disease severity, activity and response to treatment represent a great unmet need in anti-MAG PN.
Nerve damage proteins are increasingly used as disease-overarching response biomarkers in neuropathies, and have been introduced not only in the research setting but also in routine clinical care. Neurofilament light chain (NfL) is used as response biomarker by detecting axonal damage regardless of the underlying disorder, and, recently, contactin-1 (CNTN-1) has been identified as a potential biomarker for damage to the paranode (a nerve region in between the node of Ranvier and compact myelin) where the MAG protein is also present. We therefore aimed to assess whether serum NfL, and CNTN-1 levels may function as nerve damage biomarkers in anti-MAG PN.
Methods
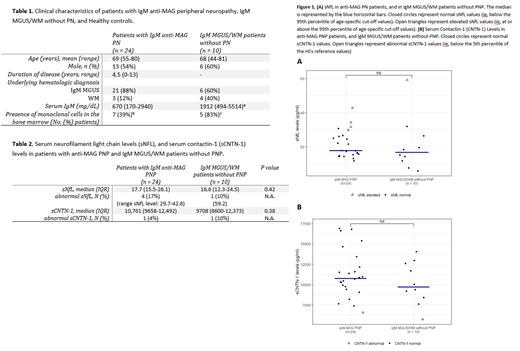
We included treatment-naïve patients with anti-MAG PN (PN) who fulfilled the following criteria: IgM paraproteinemia in the serum, presence of an IgM MAG-antibody in the serum, a clinical diagnosis of anti-MAG PN by a neurologist with expertise in peripheral nerve disorders confirmed by nerve conduction studies. As controls, treatment-naïve patients with IgM MGUS or WM without signs of neuropathy were included (non-PN). NfL levels were measured in serum using a Simoa assay. Abnormal sNfL levels were defined as at or above the 95th percentile of age-specific cut-off values in healthy controls (HCs). CNTN-1 levels were measured using the Human Magnetic Luminex Assay. Abnormal sCNTN1 levels were defined as below the 5th percentile of values in HCs. Differences between PN and non-PN groups were assessed with the Mann-Whitney U test.
Results
A total of 24 PN and 10 non-PN patients were included (Table 1). In the PN cohort, all patients had anti-MAG antibodies, with a titer of >10.000 in 22 (92%) patients. Data from 222 HCs (mean age 46 years; SD 14; range 19-98 years) were used to establish reference values. Median sNfL levels were similar between PN and non-PN patients (Table 2). Abnormal sNfL levels were present in 4 (17%) PN patients (range sNfL: 29.7-42.8 pg/mL) and in 1 (10%; 59.2pg/mL) non-PN patient (Figure 1A). Median sCNTN1 levels were also similar between PN and non-PN patients (Table 2). One (4%) patient in the PN cohort and 1 (10%) patient in the non-PN cohort had abnormal CNTN-1 levels (Figure 1B).
Conclusion
Our results do not support further studies on NfL and/or CNTN-1 in serum as potential biomarkers in anti-MAG PN, since the majority of anti-MAG PN and non-PN patients had normal levels of sNfL and sCNTN-1. In case sNfL was abnormal, levels were only modestly increased above cutoff and substantially lower compared to the high levels seen in other neuropathies.
Minnema: Alnylam: Consultancy; Celgene: Other: Travel expenses; BMS: Consultancy; Cilag: Consultancy; Janssen: Consultancy; Kite/Gilead: Consultancy. Kersten: Celgene: Research Funding; Novartis: Consultancy, Honoraria, Other: Travel support; Miltenyi Biotec: Consultancy, Honoraria, Other: Travel support; Roche: Consultancy, Honoraria, Other: Travel support, Research Funding; BMS/Celgene: Consultancy, Honoraria; Takeda: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding. Vos: Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Other: Travel reimbursement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal